11. Fully anhydrous HCl electrolysis using polybenzimidazole membranes
Kris Likit-anurak, Rembert White, Laura A. Murdock, Brian C. Benicewicz, Sirivatch Shimpalee, Benjamin H. Meekins Inter. J. of Hydrogen Ener. Just accepted
We demonstrate for the first time a fully anhydrous gas-phase hydrogen chloride electrolyzer. By taking advantage of polybenzimidazole's (PBI) low membrane resistivity in anhydrous conditions (0.87 ± 0.57 Ω-cm2 at 160 oC) and its temperature and acid environment stability, we are able to directly convert pure hydrogen chloride gas to dry hydrogen and dry chlorine at efficiencies nearing 100% at an electrolyzer temperature of 160 oC and electrolyzer potential of 1.8 V. Finally, we discuss steps to further improve this electrolysis process in terms of both electrolyzer performance and cost reduction.
10. Electrode optimization for efficient hydrogen production using an SO2-depolarized electrolysis cell
Héctor R. Colón-Mercado, Scott A. Mauger, Maximilian B. Gorensek, Cy H. Fujimoto, Aaron A. Lando, Prabhu Ganesan, Benjamin H. Meekins, Noah D. Meeks. Inter. J. of Hydrogen Ener. 47 (31), pp. 14180-14185
The hybrid sulfur (HyS) cycle offers an alternative route to hydrogen and sulfuric acid production using the SO2-depolarized electrolysis (SDE) cell. This work reports the most efficient SDE operation to date at high sulfuric acid concentrations (~60 wt%) achieved through the optimization of operating conditions and cell components. We observed that open porosity in the porous transport media (PTM) plays a significant role in SDE performance as it enables efficient acid removal from the catalyst layer. The combination of membrane electrode assembly (MEA) components, such as Sulfonated Diels Alder Poly (phenylene) (SDAPP) membranes and electrodes prepared using SGL 29BC PTM, and operating conditions (103.4 kPagauge at 125 °C) yielded electrolysis potentials <700 mV at 500 mA/cm2 and acid concentrations >60 wt%.
9. High-performance SO2-depolarized electrolysis cell using advanced polymer electrolyte membranes
Héctor R. Colón-Mercado, Maximilian B. Gorensek, Cy H. Fujimoto, Aaron A. Lando, Benjamin H. Meekins. Inter. J. of Hydrogen Ener. 47 (1), pp. 57-68
Three different proton conducting polymeric membrane materials (Nafion® 115, Nafion® 212, and sulfonated Diels-Alder polyphenylene [SDAPP]) were evaluated for use in SO2-depolarized electrolyzers for the production of hydrogen via the hybrid sulfur cycle. Their performance was measured using different water feed strategies to minimize overpotential losses while maintaining high product acid concentration. Both thin membranes (Nafion® 212 and SDAPP) showed performance superior to that of the thicker Nafion® 115. The SDAPP membrane electrode assembly (MEA) performed well at higher acid concentrations, maintaining low ohmic and kinetic overpotentials. Finally, short-term (100-h) stability tests under constant current conditions showed minimal degradation for the SDAPP and Nafion® 212 MEAs. SDAPP MEA performance approached the targets needed to make the hybrid sulfur cycle a competitive process for hydrogen production (product acid concentration ≥65 wt% H2SO4 at ≤ 0.6-V cell potential and ≥0.5 A-cm−2 current density).
8. Parametric study of operating conditions of an SO2-depolarized electrolyzer
Maximilian B Gorensek, Benjamin Meekins, Héctor Colón-Mercado, John Weidner. Inter. J. of Hydrogen Ener. 45 (43), pp. 22408-22418
Large-scale hydrogen generation is needed to satisfy both existing commercial demands and the realization of a hydrogen economy. The hybrid sulfur cycle has the potential to meet this goal without the use of fossil fuels. The ability to model and predict how the electrolyzer step of the cycle is affected by independent and interdependent variables is vital to maximizing the efficiency of the overall cycle. Our parametric study of the SO2-depolarized electrolyzer, representing one half of the overall reaction cycle, has identified desirable conditions and will help guide further development for operation that will achieve the benchmarks for economic feasibility. We discuss the interactions among the inlet flow rates, applied current, and cell temperature and pressure, on the resulting cell voltage and acid concentration in the liquid product stream. For example, we find that using a sulfonated polybenzimidazole (s-PBI) membrane and a platinum catalyst, a constant current density of either 0.5 A cm−2, 0.75 A cm−2, or 1 A cm−2 with an acid concentration of 65 wt% H2SO4 can be produced at a cell voltage of 0.59 V, 0.63 V, and 0.67 V, respectively, by maintaining a cell temperature of 130 °C, a pressure of 2 bar, and a water-to-SO2 stoichiometry of 2.77. Finally, we discuss directions for future research based on the findings in this manuscript.
7. In-situ and Ex-situ Comparison of the Electrochemical Oxidation of SO2 on Carbon Supported Pt and Au Catalysts
Benjamin H. Meekins, Anthony B. Thompson, Varsha Gopal, Bahareh A. Tavakoli Mehrabadi, Mark C. Elvington, Prabhu Ganesan, Ty A. Newhouse-Illige, Adam W. Shepard, Lawrence E. Scipioni, James A. Greer, John C. Weiss, John W. Weidner, Hector R. Colon-Meracdo. Inter. J. of Hydro. Ener. 45 (3), pp. 1940-1947
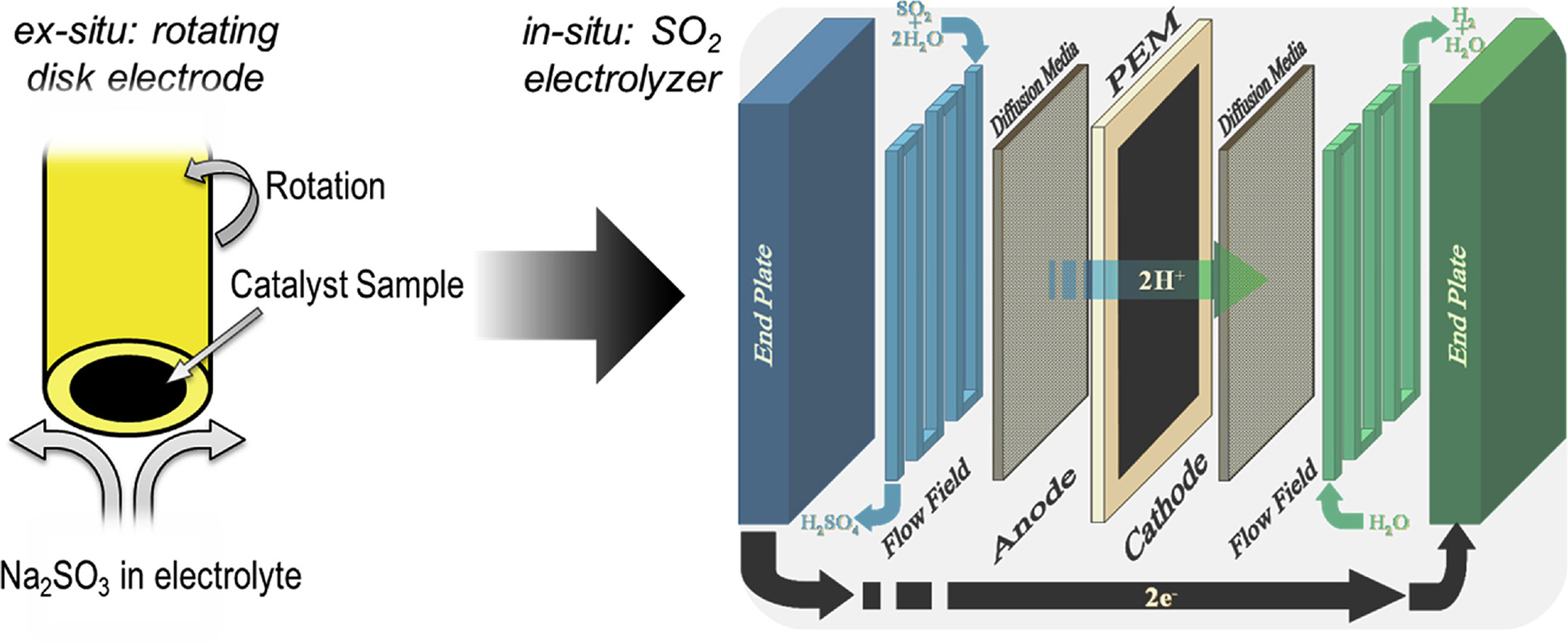 Electrochemical characterizations are performed using thin films and commercial carbon supported platinum and gold catalysts for sulfur dioxide oxidation, the primary electrochemical oxidation reaction in the Hybrid-sulfur (HyS) thermochemical process. Electrochemical evaluation of metal thin films qualitatively confirms the higher activity of Au over Pt, AuPt, Pd, and Ir for the electrochemical oxidation of SO2. Ex-situ testing, using rotating disk electrode (RDE), shows an earlier onset potential for Au/C at low sulfuric acid concentrations (C ≤ 3.5 M) and a higher turnover frequency than Pt/C at sulfuric acid concentrations ranging from 3.5 M to 9 M. In-situ electrolysis experiments using low catalyst loadings (0.1 mgAu cm−2, a factor of ≥5 lower than typical loadings) confirm that Au nanoparticles exhibit higher current densities and greater stability than Pt nanoparticles. This is consistent with the thin film screening studies, which showed higher activity with increasing gold content in AuPt thin films. This work reveals an alternative material to state-of-the-art Pt to lower the energy needs and aid the HyS cycle in reaching the target of $2/kg H2 set forth by the Department of Energy to achieve economic feasibility of large-scale hydrogen generation.
Electrochemical characterizations are performed using thin films and commercial carbon supported platinum and gold catalysts for sulfur dioxide oxidation, the primary electrochemical oxidation reaction in the Hybrid-sulfur (HyS) thermochemical process. Electrochemical evaluation of metal thin films qualitatively confirms the higher activity of Au over Pt, AuPt, Pd, and Ir for the electrochemical oxidation of SO2. Ex-situ testing, using rotating disk electrode (RDE), shows an earlier onset potential for Au/C at low sulfuric acid concentrations (C ≤ 3.5 M) and a higher turnover frequency than Pt/C at sulfuric acid concentrations ranging from 3.5 M to 9 M. In-situ electrolysis experiments using low catalyst loadings (0.1 mgAu cm−2, a factor of ≥5 lower than typical loadings) confirm that Au nanoparticles exhibit higher current densities and greater stability than Pt nanoparticles. This is consistent with the thin film screening studies, which showed higher activity with increasing gold content in AuPt thin films. This work reveals an alternative material to state-of-the-art Pt to lower the energy needs and aid the HyS cycle in reaching the target of $2/kg H2 set forth by the Department of Energy to achieve economic feasibility of large-scale hydrogen generation.
6. Combustion Synthesis and Photoelectrochemical Characterization of Gallium Zinc Oxynitrides
Austin E. Kennedy and Benjamin H. Meekins. J. Mater. Res. 33 (23), pp 3971-3978
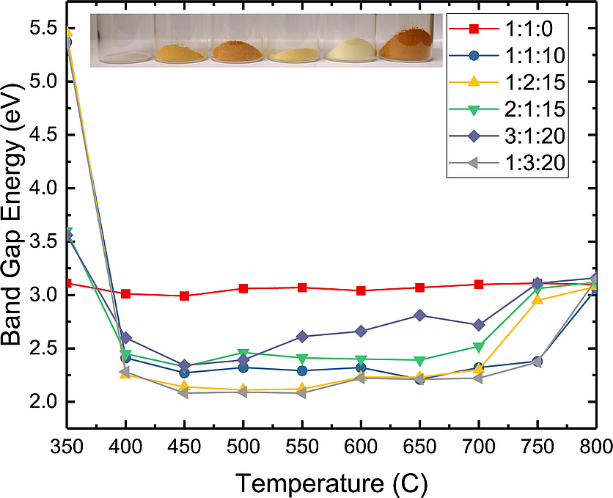 We report a rapid combustion synthesis method for producing band gap tunable gallium zinc oxynitrides, a material of interest for water splitting applications. By varying the ratio of zinc and gallium, we can tune the band gap from 2.22 to 2.8 eV. Furthermore, nitrogen can be incorporated up to nearly 50% via replacement of oxygen without the need for high temperatures or an additional ammonolysis step. X-ray photoelectron spectroscopy (XPS) and EDX analysis suggests a preferential segregation of Zn to the surface of the as-synthesized particles, though the surface Ga/Zn molar ratio in the as-synthesized particles is correlated with the Ga/Zn molar ratio of the precursor materials. Photoelectrochemical measurements show that the oxynitride powders are photoactive under both AM1.5 and visible-only (λ > 435 nm) irradiation. Hydrogen and oxygen were both evolved in half-reaction experiments under simulated AM1.5 irradiation without externally applied bias, although addition of an OER catalyst did not enhance the rate of oxygen formation, suggesting that intra- and interparticle recombination are significant.
We report a rapid combustion synthesis method for producing band gap tunable gallium zinc oxynitrides, a material of interest for water splitting applications. By varying the ratio of zinc and gallium, we can tune the band gap from 2.22 to 2.8 eV. Furthermore, nitrogen can be incorporated up to nearly 50% via replacement of oxygen without the need for high temperatures or an additional ammonolysis step. X-ray photoelectron spectroscopy (XPS) and EDX analysis suggests a preferential segregation of Zn to the surface of the as-synthesized particles, though the surface Ga/Zn molar ratio in the as-synthesized particles is correlated with the Ga/Zn molar ratio of the precursor materials. Photoelectrochemical measurements show that the oxynitride powders are photoactive under both AM1.5 and visible-only (λ > 435 nm) irradiation. Hydrogen and oxygen were both evolved in half-reaction experiments under simulated AM1.5 irradiation without externally applied bias, although addition of an OER catalyst did not enhance the rate of oxygen formation, suggesting that intra- and interparticle recombination are significant.
5. Detection of Single Metal Nanoparticle Collision Events in Non-Aqueous Media
Benjamin H. Meekins. Phys. Chem. Chem. Phys. 19 (26), pp 17256-17262.
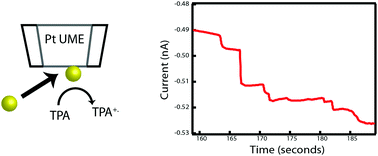 In this work, the detection of metal nanoparticle collision events in a non-aqueous solvent—here, toluene and acetonitrile—using gold nanoparticles and a platinum ultramicroelectrode (UME) is reported. The collisions were monitored by the oxidation of tri-n-propylamine (TPrA) under diffusion-dominated conditions. Based on the current response, it was observed that the current steps were indicative of a mediated Faradaic reaction. Current steps as small as 1–2 pA could be clearly observed. Larger current steps were caused by agglomeration of the nanoparticles attributed to the polarity of the mixed solvent. The experimentally observed collisions per second ranged from 0.07 to 0.51, indicating that particle agglomeration in solution occurs rapidly, reversibly and can subsequently cause rapid and, often, repeated collisions.
In this work, the detection of metal nanoparticle collision events in a non-aqueous solvent—here, toluene and acetonitrile—using gold nanoparticles and a platinum ultramicroelectrode (UME) is reported. The collisions were monitored by the oxidation of tri-n-propylamine (TPrA) under diffusion-dominated conditions. Based on the current response, it was observed that the current steps were indicative of a mediated Faradaic reaction. Current steps as small as 1–2 pA could be clearly observed. Larger current steps were caused by agglomeration of the nanoparticles attributed to the polarity of the mixed solvent. The experimentally observed collisions per second ranged from 0.07 to 0.51, indicating that particle agglomeration in solution occurs rapidly, reversibly and can subsequently cause rapid and, often, repeated collisions.
4. Compositional Screening of the Pb–Bi–Mo–O System. Spontaneous Formation of a Composite of p-PbMoO4 and n-Bi2O3 with Improved Photoelectrochemical Efficiency and Stability
Ki Min Nam, Hyun S. Park, Heung Chan Lee, Benjamin H. Meekins, Kevin C. Leonard, and Allen J. Bard. J. Phys. Chem. Lett., 2013, 4 (16), pp 2707–2710.
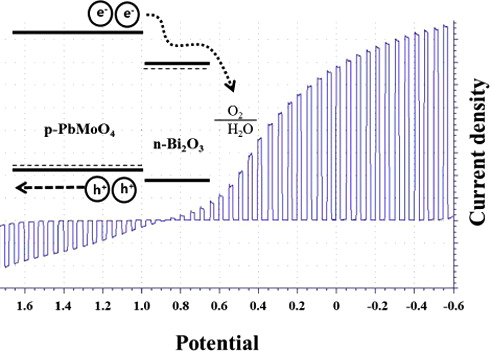 Scanning electrochemical microscopy (SECM) was used to identify more efficient p-type photocatalysts for H+ reduction in the Pb–Bi–Mo trimetal oxide system. An atomic ratio of (1:1:1) between Pb, Bi, and Mo showed higher photocurrent than other spots in screening experiments. The crystal structure of Pb–Bi–Mo oxide was studied by X-ray diffraction, indicating that a composite of p-PbMoO4 and n-Bi2O3 coexisted as a heterostructure. Photoelectrochemical performance of the p-PbMoO4/n-Bi2O3 composite electrode showed enhanced stability for the H+ and O2 reduction reactions. We propose a reaction mechanism to explain this stabilization of a p-type semiconductor.
Scanning electrochemical microscopy (SECM) was used to identify more efficient p-type photocatalysts for H+ reduction in the Pb–Bi–Mo trimetal oxide system. An atomic ratio of (1:1:1) between Pb, Bi, and Mo showed higher photocurrent than other spots in screening experiments. The crystal structure of Pb–Bi–Mo oxide was studied by X-ray diffraction, indicating that a composite of p-PbMoO4 and n-Bi2O3 coexisted as a heterostructure. Photoelectrochemical performance of the p-PbMoO4/n-Bi2O3 composite electrode showed enhanced stability for the H+ and O2 reduction reactions. We propose a reaction mechanism to explain this stabilization of a p-type semiconductor.
3. Photoactive Porous Silicon Nanopowder
Benjamin H. Meekins, Ya-Cheng Lin, Joseph S. Manser, Khachatur Manukyan, Alexander S. Mukasyan, Prashant V. Kamat, and Paul J. McGinn. ACS Appl. Mater. and Inter. 2013, 5 (8), pp 2943–2951.
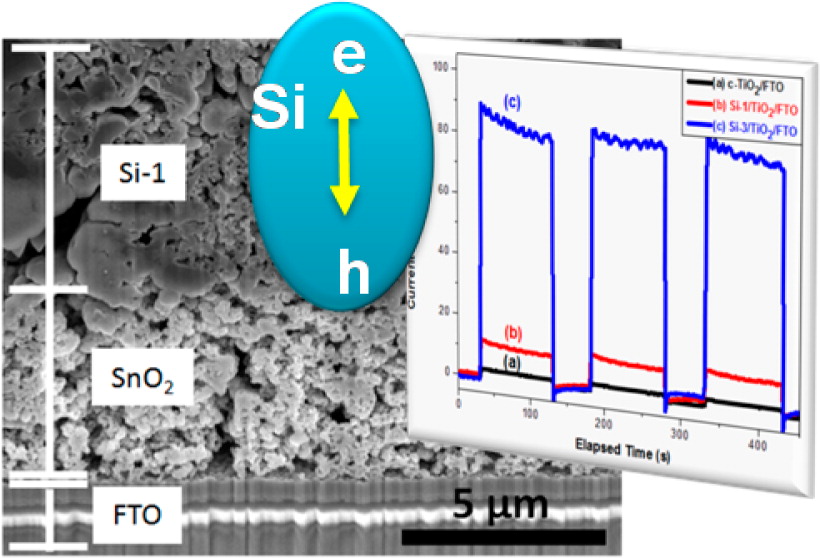 Bulk processing of porous silicon nanoparticles (nSi) of 50–300 nm size and surface area of 25–230 m2/g has been developed using a combustion synthesis method. nSi exhibits consistent photoresponse to AM 1.5 simulated solar excitation. In confirmation of photoactivity, the films of nSi exhibit prompt bleaching following femtosecond laser pulse excitation resulting from the photoinduced charge separation. Photocurrent generation observed upon AM 1.5 excitation of these films in a photoelectrochemical cell shows strong dependence on the thickness of the intrinsic silica shell that encompasses the nanoparticles and hinders interparticle electron transfer.
Bulk processing of porous silicon nanoparticles (nSi) of 50–300 nm size and surface area of 25–230 m2/g has been developed using a combustion synthesis method. nSi exhibits consistent photoresponse to AM 1.5 simulated solar excitation. In confirmation of photoactivity, the films of nSi exhibit prompt bleaching following femtosecond laser pulse excitation resulting from the photoinduced charge separation. Photocurrent generation observed upon AM 1.5 excitation of these films in a photoelectrochemical cell shows strong dependence on the thickness of the intrinsic silica shell that encompasses the nanoparticles and hinders interparticle electron transfer.
2. Role of Water Oxidation Catalyst IrO2 in Shuttling Photogenerated Holes Across TiO2 Interface
Benjamin H. Meekins and Prashant V. Kamat. J. Phys. Chem. Lett. 2011, 2 (18), pp 2304–2310.
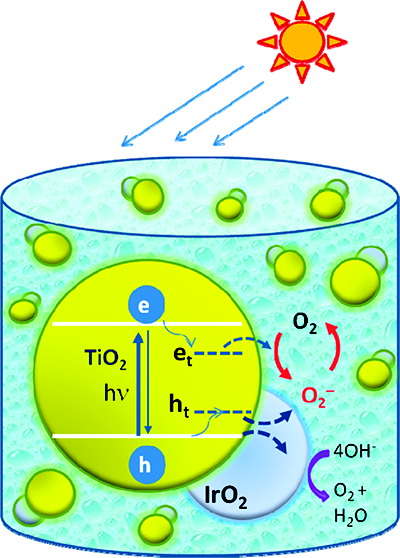 Iridium oxide, a water oxidation cocatalyst, plays an important role in mediating the hole transfer process of a UV-irradiated TiO2 system. Spectroscopic identification of trapped holes has enabled their characterization in colloidal TiO2 suspension and monitoring of the transfer of trapped holes to IrO2. Titration of trapped holes with potassium iodide yields an estimate of three holes per particle during 7 min of UV irradiation of TiO2 suspension in ethanol containing 5% acetic acid. The hole transfer to IrO2 occurs with a rate constant of 6 × 105 s-1. Interestingly, IrO2 also catalyzes the recombination of trapped holes with reduced oxygen species. The results discussed here provide a mechanistic and kinetic insight into the catalytic role of IrO2 in the photogenerated hole transfer process.
Iridium oxide, a water oxidation cocatalyst, plays an important role in mediating the hole transfer process of a UV-irradiated TiO2 system. Spectroscopic identification of trapped holes has enabled their characterization in colloidal TiO2 suspension and monitoring of the transfer of trapped holes to IrO2. Titration of trapped holes with potassium iodide yields an estimate of three holes per particle during 7 min of UV irradiation of TiO2 suspension in ethanol containing 5% acetic acid. The hole transfer to IrO2 occurs with a rate constant of 6 × 105 s-1. Interestingly, IrO2 also catalyzes the recombination of trapped holes with reduced oxygen species. The results discussed here provide a mechanistic and kinetic insight into the catalytic role of IrO2 in the photogenerated hole transfer process.
1. Got TiO2 Nanotubes? Lithium Ion Intercalation Can Boost Their Photoelectrochemical Performance
Benjamin H. Meekins and Prashant V. Kamat. ACS Nano, 2009, 3 (11), pp 3437–3446.
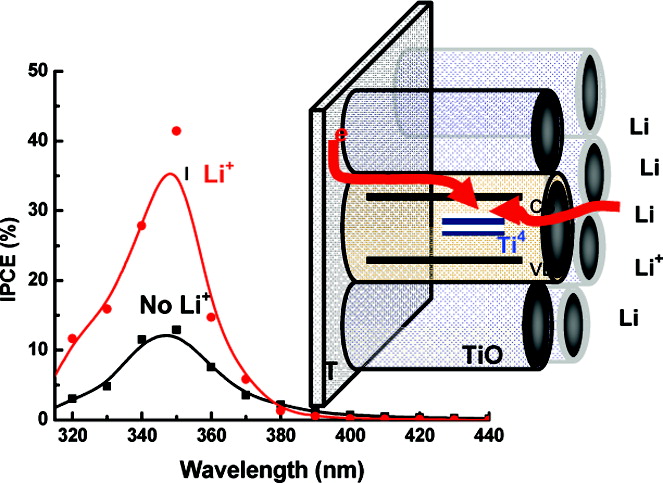 Cations such as H+ and Li+ are intercalated into TiO2 nanotube arrays by subjecting them to short-term electrochemical pulses at controlled potentials (<−1.0 V vs Ag/AgCl). The intercalation of these small cations has a profound effect toward enhancing photocurrent generation under UV light irradiation. A nearly three-fold increase in the photoconversion efficiency (IPCE) was observed upon intercalation of Li+ ions into TiO2 nanotube arrays. The intercalation process is visualized by the color change from gray to blue. Spectroelectrochemical measurements were carried out to monitor the absorption changes at different applied potentials. The analysis of the Voc decay following termination of UV light shows a significant decrease in the rate of recombination of accumulated electrons upon Li+ ion intercalation.
Cations such as H+ and Li+ are intercalated into TiO2 nanotube arrays by subjecting them to short-term electrochemical pulses at controlled potentials (<−1.0 V vs Ag/AgCl). The intercalation of these small cations has a profound effect toward enhancing photocurrent generation under UV light irradiation. A nearly three-fold increase in the photoconversion efficiency (IPCE) was observed upon intercalation of Li+ ions into TiO2 nanotube arrays. The intercalation process is visualized by the color change from gray to blue. Spectroelectrochemical measurements were carried out to monitor the absorption changes at different applied potentials. The analysis of the Voc decay following termination of UV light shows a significant decrease in the rate of recombination of accumulated electrons upon Li+ ion intercalation.